The information contained in the site is intended for Singapore healthcare professionals only. By selecting “Okay” below, you verify that you are a licensed Singapore healthcare professional.
By selecting “Okay” below, you verify that you are a licensed Singapore healthcare professional.




Despite a landscape clouded in complexity, established and emerging biomarkers are expanding our view of patient populations, and biomarker testing could provide a more comprehensive patient profile and lead to more informed decisions.


CLDN18.2=Claudin18.2; FGFR2b=fibroblast growth factor receptor 2b; HER2=human epidermal growth factor receptor 2; MSI=microsatellite instability; PD-L1=programmed death-ligand 1.
AN UNMET NEED
In Singapore, for gastric cancers, 58.2% of patients were diagnosed at Stages III-IV, where the five-year age-standardised relative survival (ASRS) rate is below 40%1.
The five-year ASRS rate for Stage IV diseases in Singapore is as low as 5%1,2, which is comparable to the overall global rate (≤10%)4


In Singapore, GC is the 8th most common cancer in men and the 10th most common cancer in women in Singapore population.3




In Singapore, mortality caused by GC is the 6th most common in men and the 7th most common in women.3




‡Locally advanced (stage II and III) and metastatic (stage IV) gastric/GEJ cancer per TNM staging classification as described in NCCN Guidelines®.4


Over 1 million new cases of G/GEJ cancers were diagnosed worldwide in 2020, making it the 5th most diagnosed cancer.5


An estimated 769,000 people died worldwide in 2020 due to G/GEJ cancers, making it the 4th most deadly cancer.5


EMERGING BIOMARKERS
help identify previously undefined subsets of metastatic gastric (mG)/ gastroesophageal junction (GEJ) cancer patients:
ESTABLISHED BIOMARKERS
are used to inform clinical decisions:
Most emerging and established biomarkers can be detected by using standard, widely accepted assays such as IHC.
EMERGING BIOMARKERS
CLDN18.2:
IHC16
FGFR2b:
IHC, NGS17,18§||
Il IHC detects FGFR2b overexpression; NGS detects FGFR2 gene amplification by ctDNA.17,18
ESTABLISHED BIOMARKERS
PD-L1:
IHC4§¶
HER2:
IHC, ISH, NGS4,19§*
MSI/MMR:
PCR, NGS/IHC4§
IHC=immunohistochemistry; ctDNA=circulating tumour DNA; ISH=in situ hybridisation; MMR=mismatch repair; NGS=next generation sequencing;
§Expression utilises IHC and PCR tests;4,16,17 amplification utilises ISH and NGS tests.4,18,19
¶Varying diagnostic assays.20
#Other ISH methods (FISH=fluorescent ISH; SISH=silver ISH; CISH=chromogenic ISH; DDISH=dual-colour dual-hapten ISH).19
Emerging biomarkers are highly prevalent among mG/GEJ biomarkers.
Biomarker prevalence estimates from select studies are reported below. Prevalence data can vary amongst studies due to tumour heterogeneity, differences in patient population, clinical trial methodology, and diagnostic assays used.13,21–23
EMERGING BIOMARKERS
CLDN18.216,21
(high expression)**
36%
FGFR2b22
(positive)
30%
** 2+/3+ staining by IHC in ≥75% of tumour cells.21
ESTABLISHED BIOMARKERS
PD-L123,24
(variable due to multiple factors)††
CPS ≥1: 67-73%
CPS ≥5: 29-31%
CPS ≥10: 16-18%
HER213
(Positive)
22%
MSI25
(MSI-high)
4%
CPS=combined positive score.
††PD-L1 prevalence at various CPS thresholds is still being explored. Data are from a randomised controlled trial and a real-world, retrospective medical records study.23,24
Timeline of targetable biomarker discoveries26–30


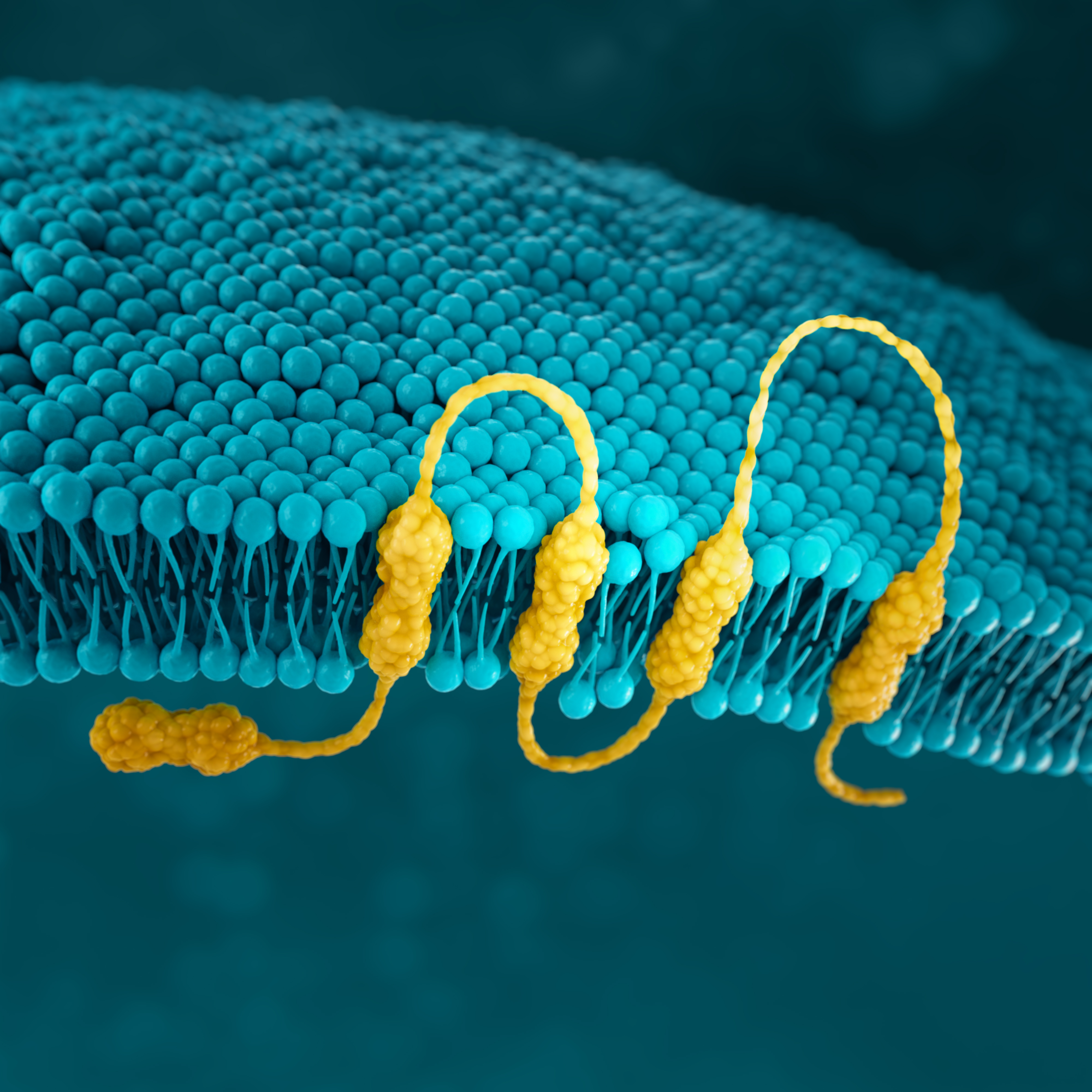
CLDN18.2
Claudins are a family of transmembrane proteins7,29:
Claudins are a major component of epithelial and endothelial tight junctions, which are involved in controlling the flow of molecules between cells.7,9
Claudins are present throughout the body, but two specific isoforms of CLDN18 are localised to certain tissue types7,29:
Preclinical studies have shown that CLDN18.2 may become more exposed and accessible to antibodies as gastric tumours develop.7,32,33
CONFINED IN HEALTHY TISSUE
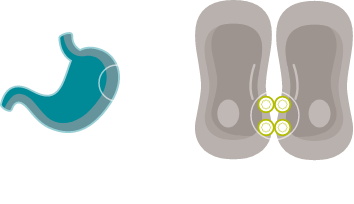
In gastric epithelial cells, CLDN18.2 is typically buried in the tight junction supramolecular complex.7,9,33
It functions to regulate selective barrier properties and contributes to cell-to-cell epithelial adhesion.7–10
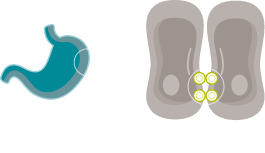
EXPOSED IN TUMOURIGENESIS


Malignant transformation leads to polarity disruptions and structure loss.32,33 As a result, CLDN18.2 may be more exposed and accessible to antibodies.7,32,33
RETAINED DURING TRANSFORMATION


The presence of CLDN18.2 is retained throughout malignant transformation, both in the primary tumour site and metastatic disease.7,32
Although not present in healthy tissues beyond gastric epithelial cells, CLDN18.2 may be overexpressed in oesophageal, pancreatic, lung, and ovarian tumours as well.7
While approximately 70% of locally advanced and mG/GEJ cancers express CLDN18.2 (at any level), recent studies have shown approximately 36% of mG/GEJ patients are CLDN18.2 positive (high expression).21
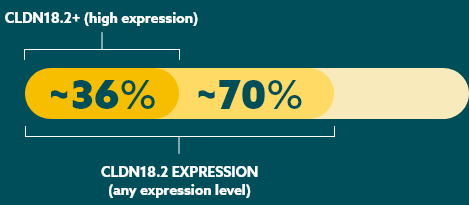

Few patients with mG/GEJ cancer who are CLDN18.2+ (high expression) also test positive for other biomarkers.21
When evaluating the relationship between CLDN18.2 and other biomarkers, current data suggest there is limited overlap.
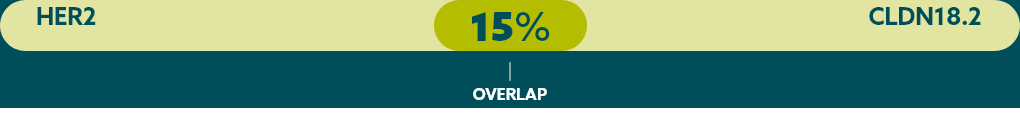

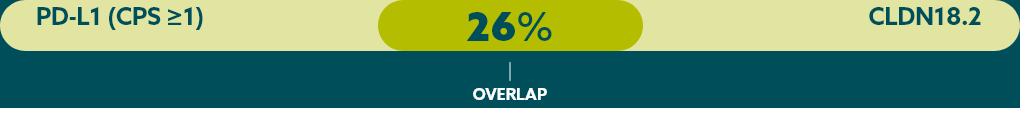

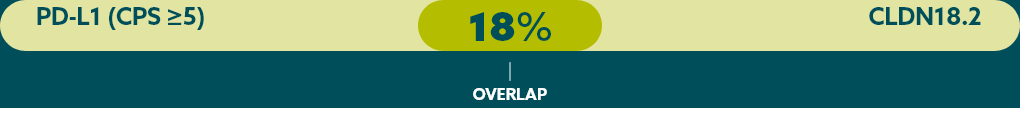

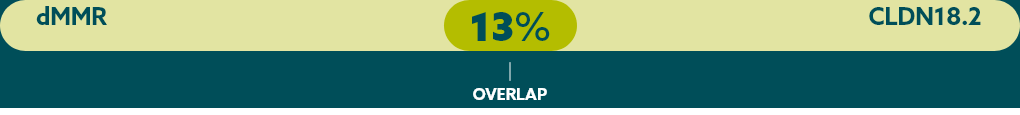

* Study population was limited to 350 Caucasian patients with mG/GEJ cancer, of which 117 patients had high expression of CLDN18.2. FGFR2b was not evaluated in this study.
CLDN18.2 is expressed in both diffuse-type tumours and intestinal-type tumours.16
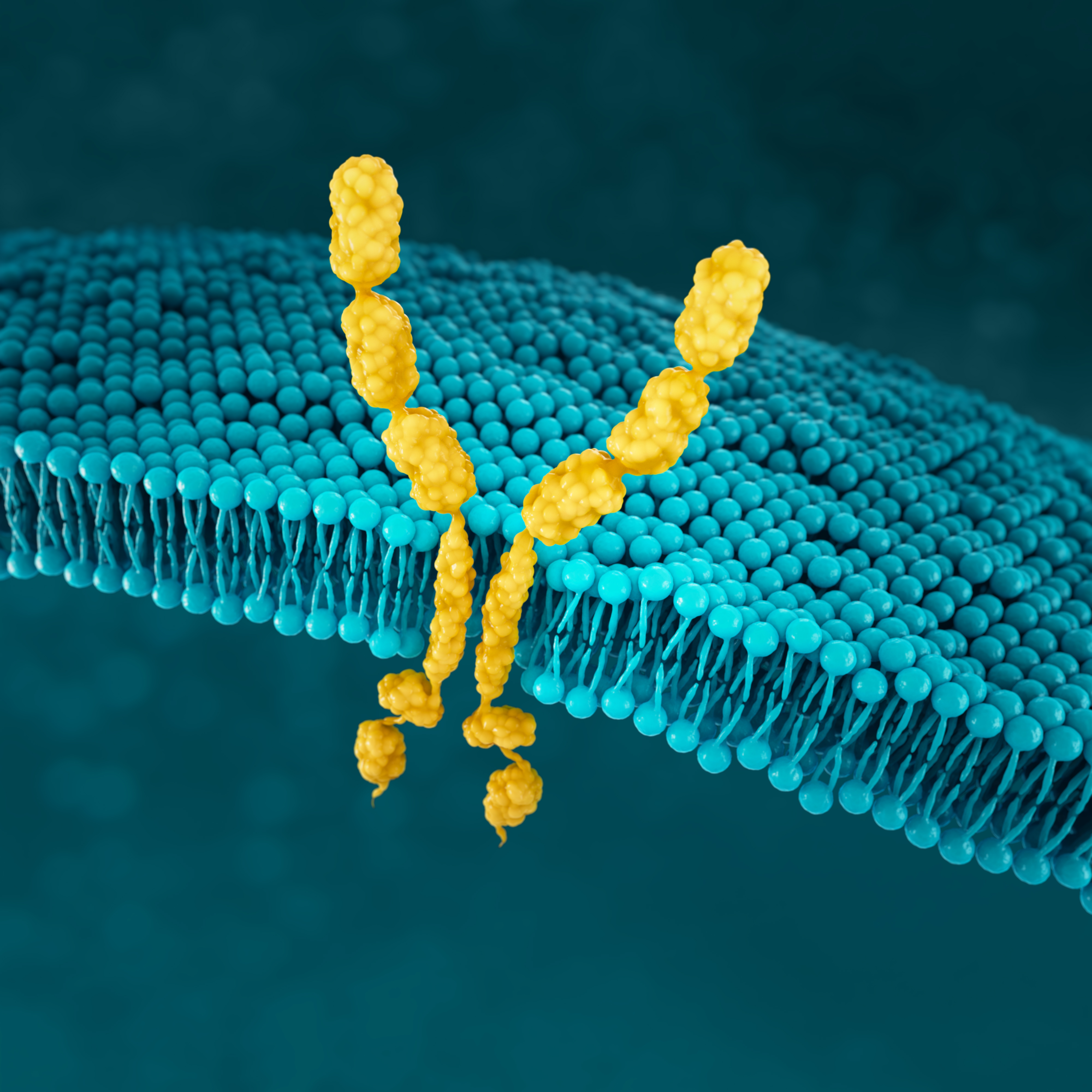
FGFR2b
FGFR signalling has a key role in many biological processes, but it also offers important information about how cancer may develop.35,36
FGFR2b positivity can be observed in 30% of mG/GEJ cancers.22


FGFR2b positivity: FGFR2b overexpression (IHC) and/or gene amplification by ctDNA (NGS)
Detecting FGFR2b can be done with the following tests17,18:
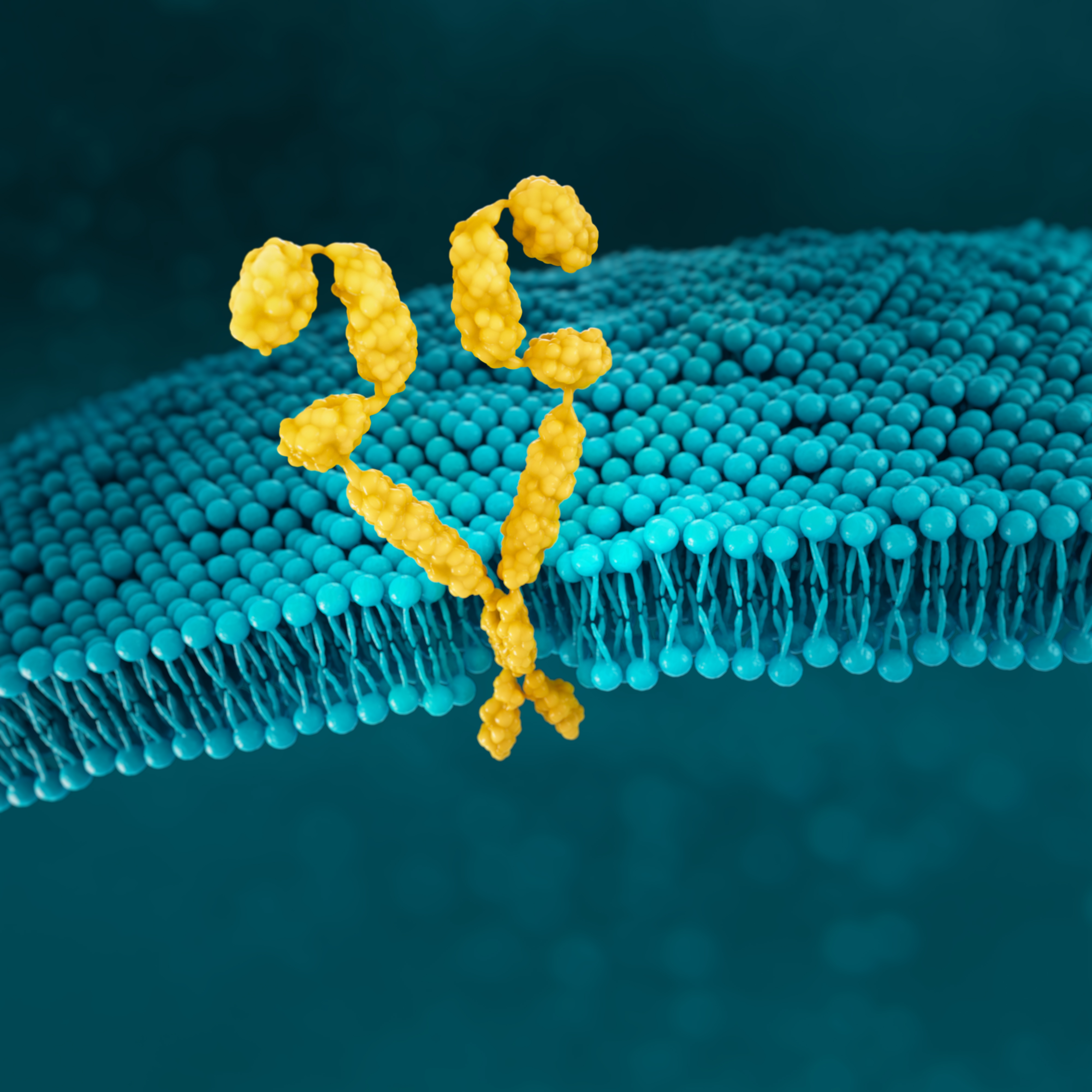
HER2
HER2 (human epidermal growth factor receptor 2) is a receptor-tyrosine kinase that is overexpressed and/or amplified in mG/GEJ cancer.11
HER2 is a proto-oncogene that is involved in signaling pathways, which leads to cell growth and differentiation.19
HER2 positivity has been reported in 22% of advanced G/GEJ cancers.13


HER2 positivity: overexpression (IHC3+) and/or gene amplification (FISH-positive)
Detection of HER2 may be done with IHC, ISH methods and NGS, and is generally more associated with intestinal type tumours. 4,13,19*
*IHC/ISH should be considered first, followed by additional NGS testing as appropriate.4

MSI
MSI is associated with genomic instability and increased susceptibility to tumour development.11
Microsatellites are repeated sequences of nucleotides in DNA.14
MSI-H has been reported in 4% of advanced G/GEJ cancers.25


Detection of MSI and MMR is typically assessed with various methods.4
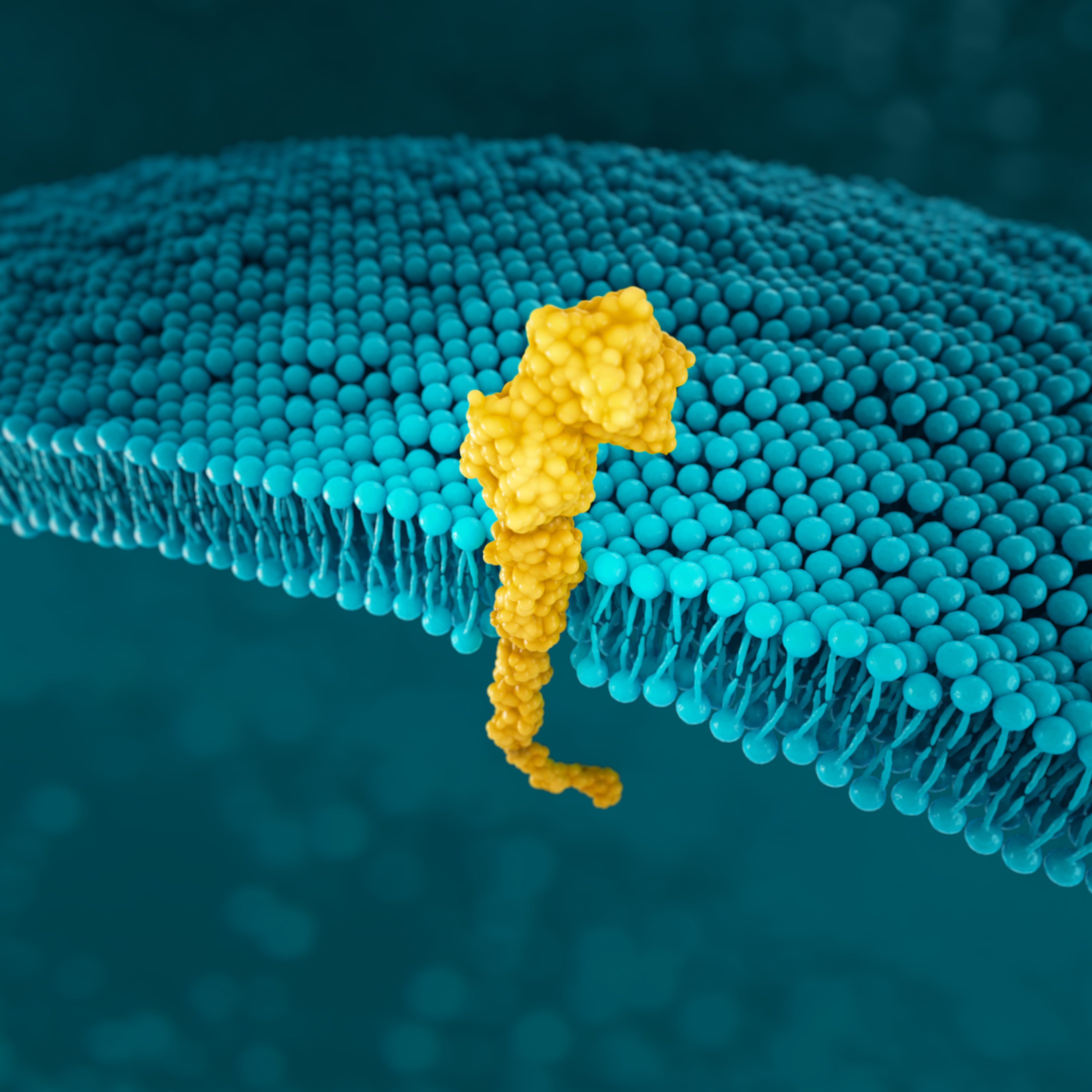
PD-L1
PD-L1 (programmed death-ligand 1) is a transmembrane protein that may be expressed on various tumor cells and/or immune cells.40
Prevalence of PD-L1 has been reported for several positivity thresholds throughout various studies23,34*†:






*Study population was limited to 592 patients with locally advanced or mG/GEJ cancer who experienced disease progression after first-line therapy with a platinum and fluoropyrimidine.24
†Study analysed 56 specimens from therapy-naïve biopsies from German patients with primarily non-metastatic gastric adenocarcinoma.23
PD-L1 expression is detected using IHC.4
SUMMARY
Guidelines for mG/GEJ cancer support the use of biomarkers to help support using biomarkers to help map the path forward for patients.4,43
Biomarker testing provides more insight into advanced mG/GEJ cancer as more biomarkers are discovered.
**IHC/ISH should be considered first, followed by additional NGS testing as appropriate.4
As biomarker research continues, it expands our view of the patient population, reveals more information about the mG/GEJ cancer landscape, and helps inform clinical decisions.
To submit a question or for any other queries, please contact jason.tang@astellas.com or click here to contact our Medical Information Team.
Stay up to date
By signing up below to receive more information, you will receive helpful email updates about emerging biomarkers in metastatic gastric cancer, and you may be contacted by a local Astellas Team Member. Please note that fields marked with (*) are required.
You will now receive email updates about emerging biomarkers in mG/GEJ cancer, and you may be contacted by an Astellas representative.
REFERENCES
This Website is established and operated by Astellas Pharma Inc. (hereinafter referred to as Astellas Pharma). We would like to request users to read the following Terms of Use carefully before using this Website. By using the Website, users are regarded as having agreed to abide by the following Terms of Use and all the related laws and regulations.
1. Environment for use
The following environment is recommended for browsing this Website. Some of the site many not be accessed in environments other than those recommended. Please note that even under the recommended environment some contents may not be usable due to special technical materials.
OS·Web browser
JavaScript
JavaScript is used in this Website. It is recommended to change the settings of your browser to enable JavaScript in order to easily use all contents.
Cookie
In this Website, information called Cookies are stored in the PC used to browse this site, for the purpose of providing better content. The cookies used in this site do not infringe on your privacy or PC environment. It is possible to prohibit the use of cookies by changing the settings of your browser. However, please note that some contents or functions may become unavailable due to such a change.
Use of Cookies
*As of April 2018
Cookies are used also for statistics analysis on the status of the use of this Website and measurement of the effect of advertisement.
About TLS security
This Website uses TLS(Transport Layer Security) encrypted transmission for all pages in order to increase security level of the transactions involving private information.
2.Copyright
All the contents included in this Website are the property of Astellas Pharma and providers of respective contents, which are protected by copyright laws, various treaties and other laws and regulations in each country. The use of this Website is restricted to personal use for non-commercial purposes, and other extent approved by laws and regulations. You are not allowed to download and/or duplicate contents beyond that extent.
3.Trademarks, etc.
The rights of trademarks, service marks, business names, designs, etc., indicated within this Website are lawfully protected. Unauthorized use of them and other acts of piracy are prohibited.
4. Precautions related to HTML
DOCTYPE declaration
This Website is made by using a coding method complying with HTML5. However, please understand that some contents have been ported by maintaining the XHTML 1.0 Strict, HTML 4.01 Transitional format.
Package and modules
Please understand that some package and modules unauthorized for source alteration, due to copyright and other reasons, do not comply with the syntax of XHTML 1.0 Strict.
5.Contents
This Website includes product information of Astellas Pharma. Such information is not necessarily matching with that of products marketed outside Japan, as the information is based on products marketed within Japan. Many of the products published in this Website are ethical pharmaceuticals requiring prescription by physicians, etc. The purpose of their contents is not to publicize or advertise the efficacy of the products.
This Website does not intend to provide advice or services that should be carried out by physicians, chemists and other medical practitioners or does not act as their substitute.
Please refer to Cautionary Statement on the page of Investor Relations.
6.Services and contents
Astellas Pharma is paying its utmost attention to information published in this Website. However, it cannot guarantee all of its accuracy, usability and soundness of the contents, as well as safety of being free from errors and/or virus infection, etc. Astellas Pharma is not responsible for any damage caused by the use or existence of this Website or information published on this Website.
7.Revisions to and availability of the Website
Astellas Pharma reserves the right to revise the contents of information published on this Website or to close it down without providing any advance notification.
8.Links
If you wish to place a link to this Website, please contact Astellas Pharma beforehand to obtain its approval. Astellas Pharma assumes no responsibility for Websites of third parties placing a link to/from this Website.
9.Other
These Terms of Use are governed by the laws of Japan and should be interpreted in the light of such laws. Any dispute relating to these Terms of Use is to be filed with the Tokyo District Court as the exclusive court of jurisdiction for the initial hearing.